
Electron configuration of oxygen ion Lousiana
The electronic configuration of oxygen is- 1s22s22p4 Note:- For writing the electronic configuration of elements, the Aufbau Principle is used. In Aufbau Principle, the electrons are filled according to the increasing energy level of orbitals.

Electronic configuration of the oxygen atom Download Scientific Diagram
In this video we will write the electron configuration for O 2-, the Oxide ion. We'll also look at why Oxygen forms a 2- ion and how the electron configurati.

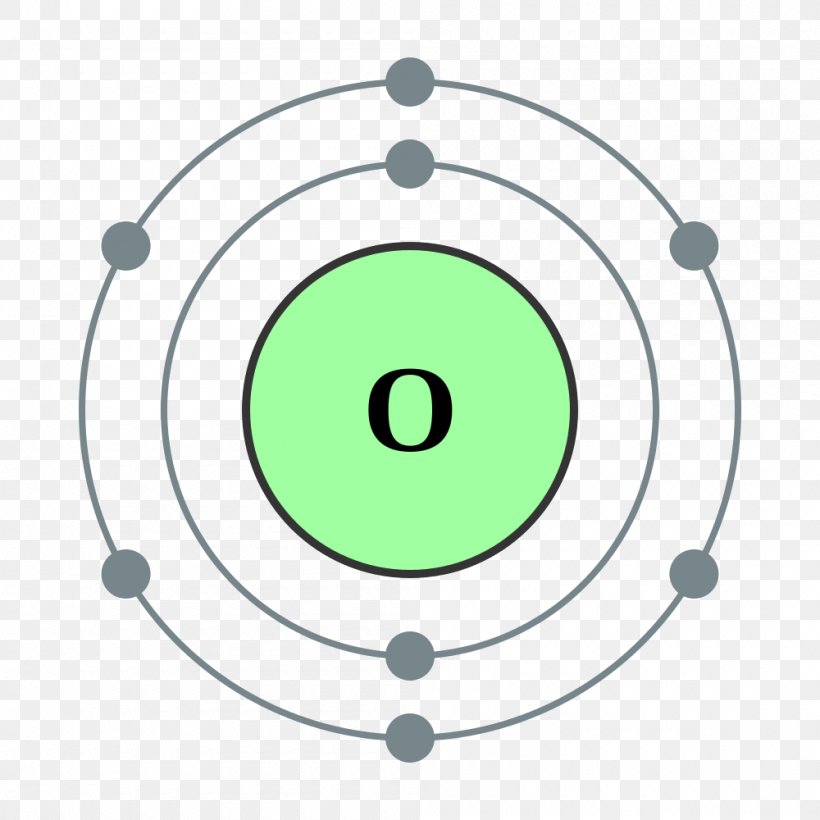
Oxygen Bohr Model (Diagram, Steps To Draw) Techiescientist
Electron configuration notation provides us with information about the basic energy levels and sublevels that electrons occupy. Ground state means that the atom has the lowest energy allowed. The electron configuration is responsible for many physical and chemical properties of an element.

FileElectron shell 008 Oxygen.svg Wikimedia Commons Atom diagram, Electron configuration
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure 6.25 ): The number of the principal quantum shell, n,
Oxygen Electron Configuration (O) with Orbital Diagram
Based on the order of fill above, these 8 electrons would fill in the following order 1s, 2s and then 2p. So Oxygen's electron configuration would be O 1s 2 2s 2 2p 4. Special Cases. Configurations of ions present a special case of electron configuration and also demonstrate the reason for the formation of those ions in the first place.
Bohr Model Oxygen Chemical Element Atomic Number, PNG, 1000x1000px, Bohr Model, Area, Atom
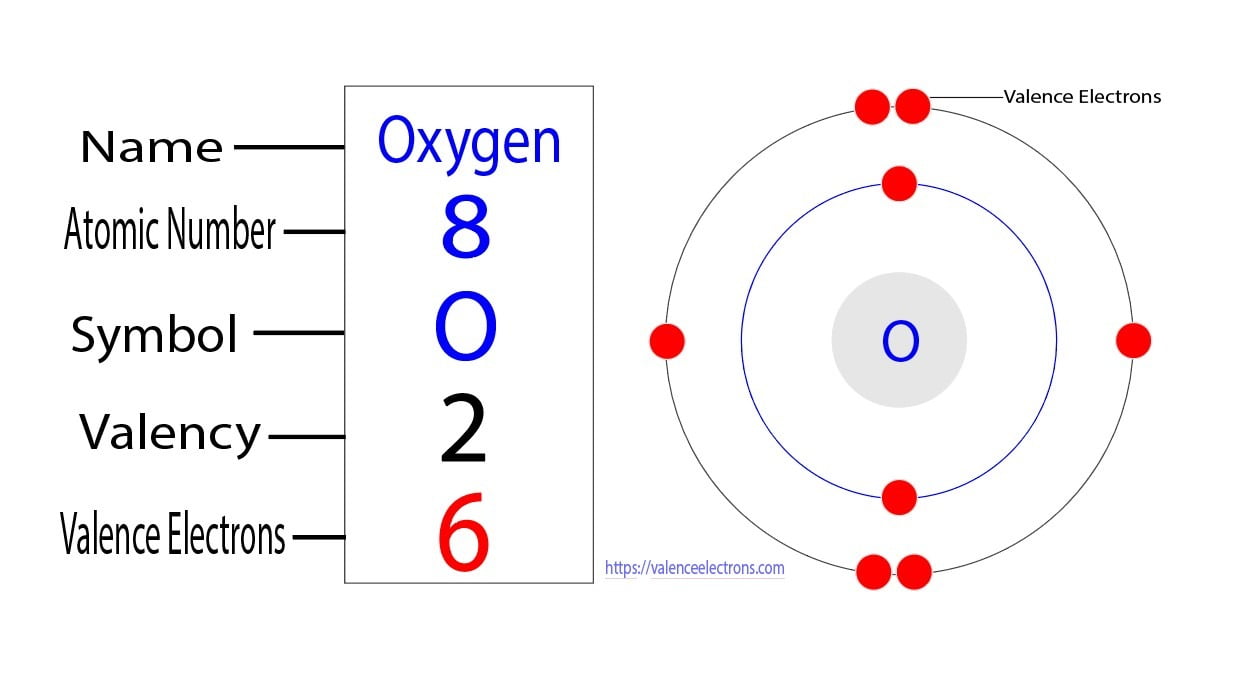
The arrangement of electrons in oxygen in specific rules in different orbits and orbitals is called the electron configuration of oxygen. The electron configuration of oxygen is [ He] 2s 2 2p 4, if the electron arrangement is through orbitals. Electron configuration can be done in two ways. Electron configuration through orbit (Bohr principle)

Electron Configuration for Oxygen (O, O2 ion)
Ground State Electron Configuration of Oxygen. The way electrons are arranged in oxygen is shown by the numbers 1s^2, 2s^2, 2p^4. This tells us how many electrons are in each part. Let's break it down and explain it more simply. Oxygen has eight electrons. The first energy level can hold two electrons, and oxygen has two at this level.

What is the Electron Configuration of Oxygen Archives Dynamic Periodic Table of Elements and
A step-by-step description of how to write the electron configuration for Oxygen (O). In order to write the O electron configuration we first need to know t.
Oxygen(O) electron configuration and orbital diagram (2022)
About Transcript Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as 2s²2p⁴. Created by Sal Khan. Questions Tips & Thanks
How to Find the Valence Electrons for Oxygen (O)?
The electron configuration of an oxygen atom [He] 2s 2 2p 4 suggests that neutral oxygen atoms can achieve an octet of valence electrons by sharing two pairs of electrons to form an O=O double bond, as shown in the figure below. According to this Lewis structure, all of the electrons in the O 2 molecule are paired.
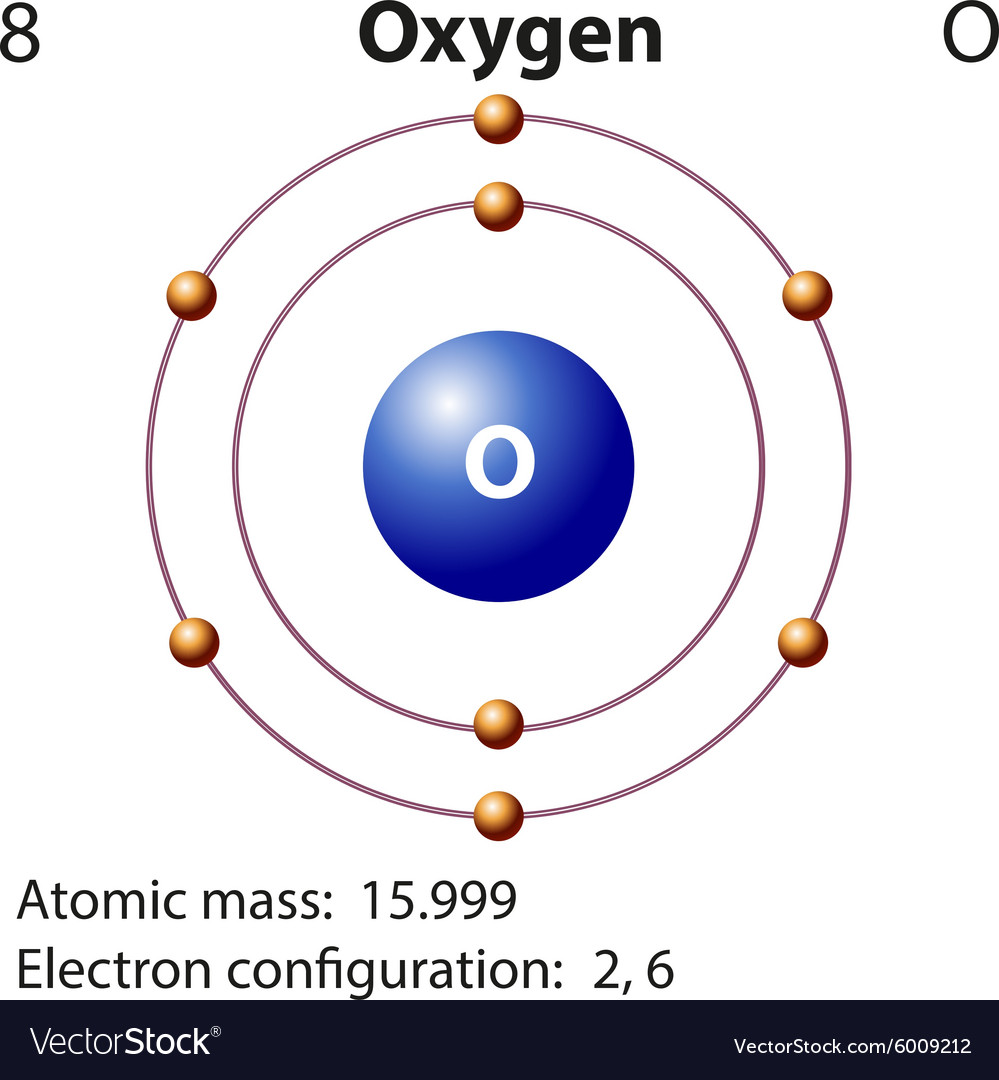
Diagram representation element oxygen Royalty Free Vector
In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic table gives the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3.

Electron Configuration Of Oxygen In Ground State
Electron configurations are a simple way of writing down the locations of all of the electrons in an atom. As we know, the positively-charged protons in the nucleus of an atom tend to attract negatively-charged electrons.
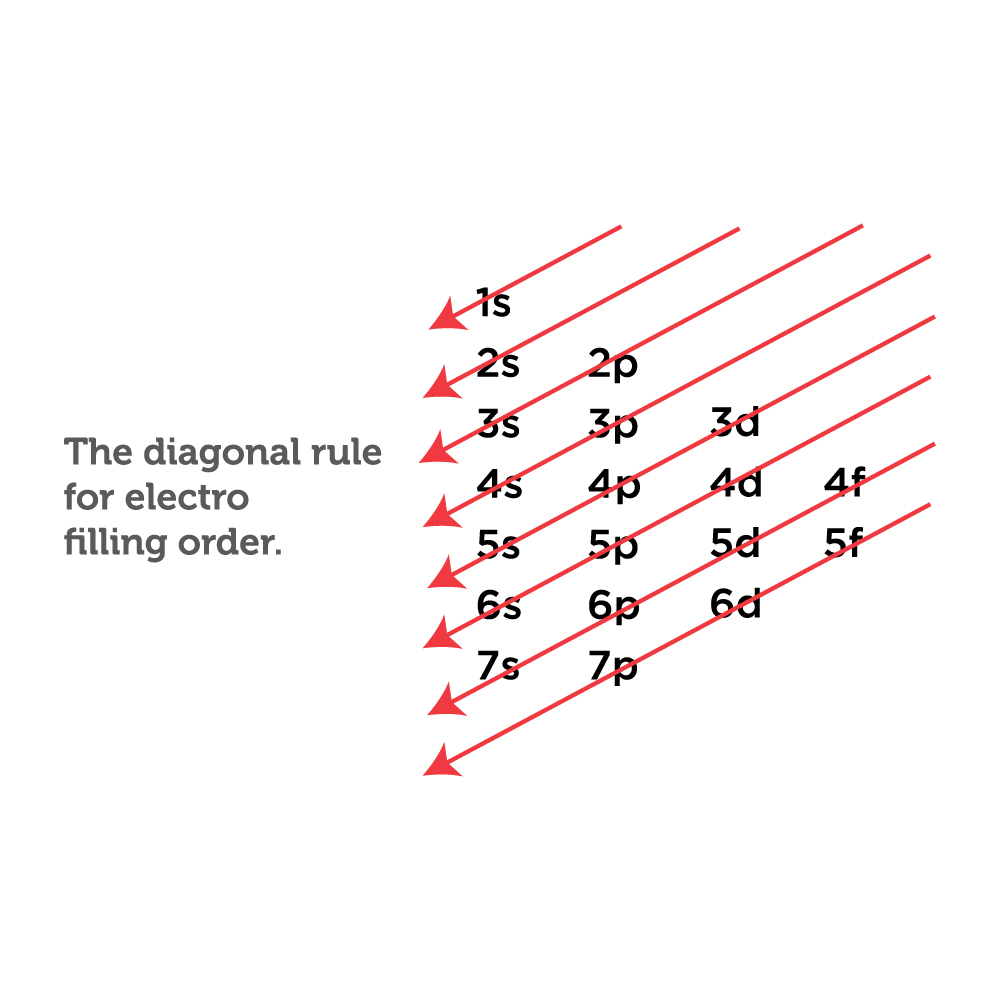
How to Write Ground State Electron Configuration in Chemistry
If we look at the element after nitrogen in the same period, oxygen (Z = 8) its electron configuration is: 1s 2 2s 2 2p 4 (for an atom). Oxygen has one more electron than nitrogen and as the orbitals are all half filled the electron must pair up. Occupation of Orbitals.

√ Electron Configuration Of Oxygen Ion Lir Stardust
Electronic configuration: The arrangement of electrons into the orbitals of an atom using some fundamental principle is called its electronic configuration. Electronic configuration of Oxygen: The electronic configuration of Oxygen is 1 s 2 2 s 2 2 p 4. Oxygen requires two electrons to attain noble gas configuration. Suggest Corrections 24

The electron configuration of oxygen is 1s2,2s2 2p4. Science chemistry, Electron configuration
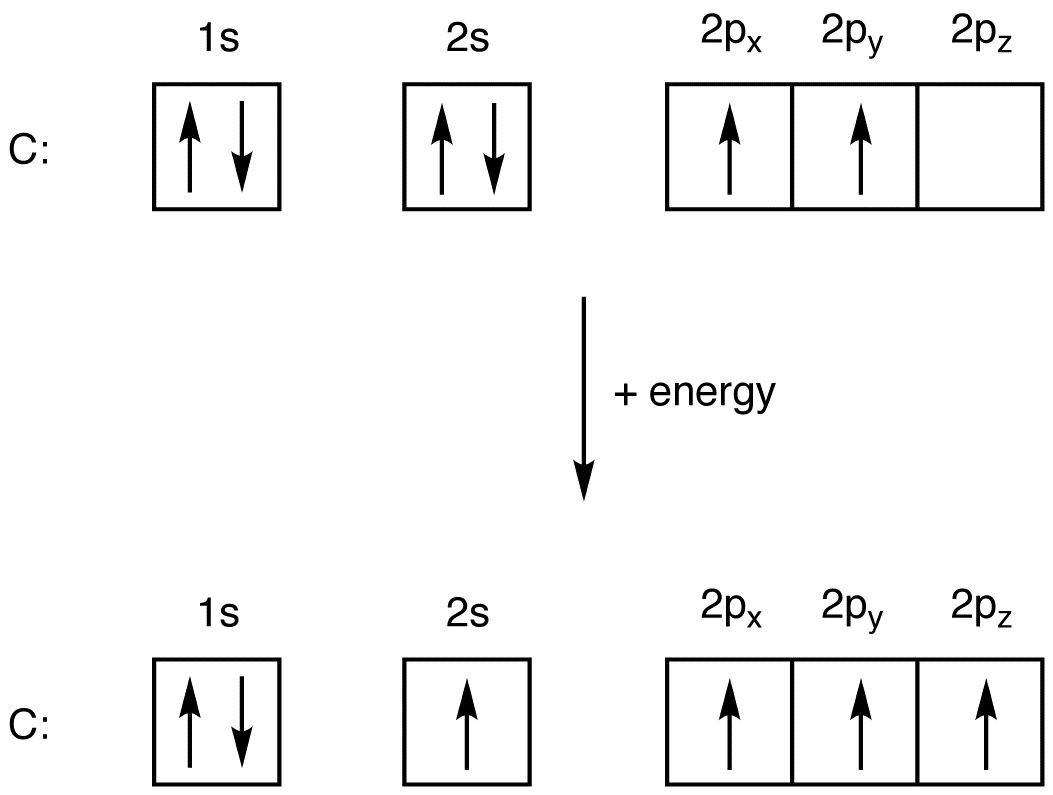
Example 1.6.3 1.6. 3: Carbon and Oxygen. Consider the electron configuration for carbon atoms: 1s 2 2s 2 2p 2: The two 2s electrons will occupy the same orbital, whereas the two 2p electrons will be in different orbital (and aligned the same direction) in accordance with Hund's rule. Consider also the electron configuration of oxygen.

Oxygen Atom Science Notes and Projects
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure 3.1.2 3.1. 2 ): The number of the principal quantum shell, n, The letter that designates the orbital type (the subshell, l ), and